Define The Octet Rule
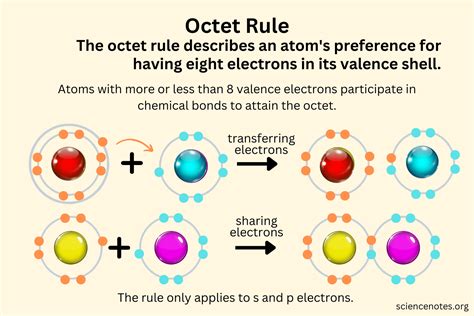
The octet rule is a fundamental concept in chemistry, particularly in the field of chemical bonding. It states that atoms tend to gain, lose, or share electrons to achieve a stable electronic configuration similar to that of a noble gas, which typically has eight valence electrons.
This rule is derived from the understanding of atomic structure and the behavior of electrons in orbitals. Atoms seek stability by filling their outermost energy levels, or valence shells, with electrons. The octet rule suggests that having eight valence electrons leads to a more stable and energetically favorable state.
Understanding Atomic Structure
To grasp the octet rule, we must first explore the structure of an atom. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in different energy levels or shells. These shells can accommodate a specific number of electrons, and the outermost shell, known as the valence shell, plays a crucial role in chemical bonding.
The first energy level, or K-shell, can hold a maximum of two electrons. The second level, the L-shell, can accommodate up to eight electrons. As we move further away from the nucleus, the subsequent shells (M, N, O, and P) can hold more electrons, but for simplicity, we often focus on the valence shell, which is the last shell to be filled.
Now, let's delve into the significance of the octet rule and how it influences chemical bonding.
The Octet Rule and Chemical Bonding
The octet rule is particularly relevant when atoms form chemical bonds. Atoms strive to achieve a stable electronic configuration, and having eight valence electrons is often the most stable arrangement. This stability is attributed to the distribution of electrons in the valence shell, which minimizes the atom's energy and maximizes its stability.
Atoms can achieve an octet of valence electrons through various mechanisms:
- Ionic Bonding: In ionic bonding, atoms transfer electrons to achieve a noble gas configuration. For example, a metal atom like sodium (Na) loses an electron to become a sodium ion (Na+), while a non-metal atom like chlorine (Cl) gains an electron to become a chloride ion (Cl-). This results in both ions having a full valence shell, satisfying the octet rule.
- Covalent Bonding: Covalent bonding involves the sharing of electrons between atoms. Atoms with fewer than four valence electrons tend to share electrons with other atoms to fill their valence shells. For instance, hydrogen (H) atoms, which have one valence electron each, can share their electrons with another hydrogen atom to form a stable H2 molecule, where each hydrogen atom has a full valence shell.
- Metallic Bonding: In metallic bonding, atoms of metallic elements share their valence electrons freely with neighboring atoms. This sharing creates a "sea of electrons" that holds the atoms together. While metallic bonding doesn't directly lead to an octet, it allows metals to exhibit unique properties like conductivity and malleability.
The octet rule provides a simplified explanation for the behavior of atoms in chemical bonding. However, it's important to note that there are exceptions and variations to this rule, especially for atoms in the transition metal series and those with odd numbers of electrons.
Exceptions to the Octet Rule

While the octet rule is a useful guideline, it does not apply universally. Here are some notable exceptions:
- Atoms with Odd Numbers of Electrons: Atoms like nitrogen (N) and oxygen (O) have five and six valence electrons, respectively. These atoms often form compounds with other atoms, leading to an odd number of electrons in the molecule. In such cases, the octet rule is not strictly followed.
- Transition Metals: Transition metal atoms, found in the d-block of the periodic table, often exhibit different bonding behaviors. They can expand their valence shells to accommodate more than eight electrons, forming compounds with various coordination numbers.
- Hypervalent Molecules: Certain molecules, such as phosphorus pentachloride (PCl5) and sulfur hexafluoride (SF6), have central atoms with more than eight valence electrons. These molecules violate the octet rule but are stabilized by other factors, such as the presence of d-orbitals.
Despite these exceptions, the octet rule remains a valuable tool for understanding and predicting chemical bonding patterns, especially for main-group elements.
Applications of the Octet Rule

The octet rule has numerous applications in chemistry and is particularly useful in the following areas:
- Predicting Chemical Formulas: By knowing the valence electrons of atoms and applying the octet rule, we can predict the likely chemical formulas of compounds formed between different elements.
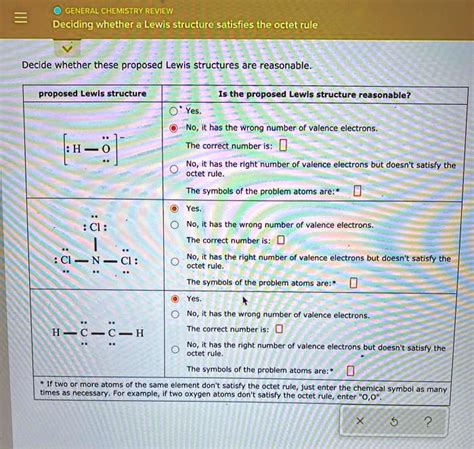
- Understanding Lewis Structures: Lewis structures, also known as electron dot diagrams, are visual representations of the distribution of electrons in molecules. The octet rule guides the placement of electrons to create stable structures.
- Explaining Chemical Properties: The octet rule helps explain why certain elements exhibit specific chemical properties. For instance, the reactivity of metals and non-metals can be attributed to their desire to achieve a stable electronic configuration.
- Teaching Chemistry: The octet rule is a fundamental concept taught in introductory chemistry courses. It provides a simple yet powerful framework for understanding atomic behavior and chemical bonding.
In conclusion, the octet rule is a fundamental concept in chemistry that explains the behavior of atoms in chemical bonding. While it has its limitations and exceptions, it remains a valuable tool for predicting and understanding the stability of chemical compounds. By gaining a deeper understanding of the octet rule, we can explore the fascinating world of chemical bonding and its applications in various scientific disciplines.
What is the octet rule, and why is it important in chemistry?
+The octet rule states that atoms tend to gain, lose, or share electrons to achieve a stable electronic configuration with eight valence electrons. It is important because it helps explain the behavior of atoms in chemical bonding and provides a foundation for understanding the stability of chemical compounds.
Are there any exceptions to the octet rule?
+Yes, there are exceptions. Atoms with odd numbers of electrons, transition metals, and hypervalent molecules often violate the octet rule. These exceptions highlight the complexity of chemical bonding and the need for a more nuanced understanding of atomic behavior.
How does the octet rule apply to ionic bonding?
+In ionic bonding, atoms transfer electrons to achieve a noble gas configuration. This results in both ions having a full valence shell, satisfying the octet rule. For example, sodium (Na) loses an electron to become Na+, and chlorine (Cl) gains an electron to become Cl-, both achieving an octet.

